Translate AI Research from the Bench to Bedside
Building an Distributed AI Lab to perfect privacy-preserving training of AI applications represents a necessary and important step towards achieving the ultimate goal of the Pediatric Moonshot. But building this critical infrastructure, alone, is insufficient. What is also needed is a major shift in how research makes its way from the lab bench to bedside. Today, far too many research projects are conducted in isolation by researchers whose ability to train neural networks is constrained by access to small local data sets, with results shared in the form of research papers.
Unfortunately, that’s generally where any new insights or learning ends — in a research paper, with no impact on patients. Seeing a similar problem in pharmaceuticals and medical devices, translational medicine emerged as a discipline to eliminate the barriers between the research bench and the bedside. While translational medicine has been applied to drugs and devices, there has been little work in translating software innovations.
One of the important uses of a distributed AI cloud service is to provide the platform to translate AI innovations from research to the bedside. We call the three-step, translational, AI process the Chang Method in honor of Dr. Anthony Chang, who inspired the Pediatric Moonshot.
Step 1: Take the AI algorithms developed on small data sets and implement it as a distributed AI application. This implementation would include using edge data services to access the usual data (e.g. MRI, Ultrasound, EEG, blood analysis), as well as a clinical user interface.
Step 2: Deploy the AI application to the in-the-building edge cloud in all 500 children’s hospitals. Now measure the accuracy.
Step 3: Improve accuracy. Now the AI application has access to a large diverse data set use federated learning to learn while preserving privacy.
Rather than simply presenting our description of the Chang Method in abstract form, let’s now apply it to an actual, real-world scenario.
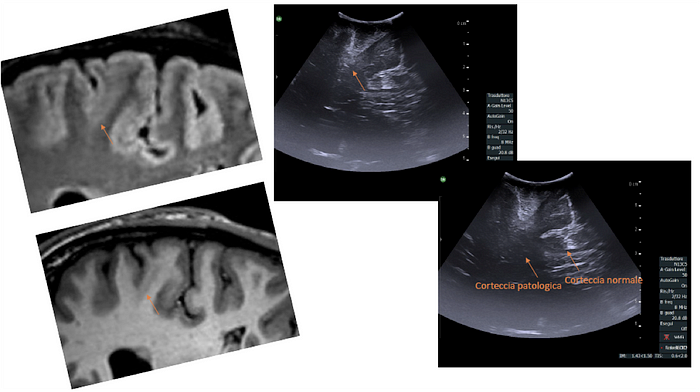
Let’s start with a child in the southern United States whom we previously introduced. Having had seizures since 18 months of age, there was no significant span of time in his life when he was seizure-free. At age twelve, he was still experiencing multiple seizures a day lasting 10–20 seconds each. Those seizures he experienced while sleeping (averaging three a night) included waking and screaming. Post-seizure, he cried, was tired, and became irritable. While the key to identifying Focal Cortical Dysplasia (FCD), the disorder he was eventually diagnosed with having, can be found in an MRI scan of the brain. Take a look at both the MRI and ultrasound image of the lesion at the beginning of the article. Unfortunately for this boy, both of his initial MRIs were inaccurately read as normal, and appropriate treatment remained elusive. With only 2500 of the cases in the US in a year, it’s not surprising that a single clinician could miss this diagnosis.
Since this child’s FCD was not identified as the underlying cause of his seizures, instead of the necessary surgical treatment, he was put on at least 10 drugs, which were each subsequently stopped due to their ineffectiveness and side effects. Recently his FCD was finally diagnosed. Surgery to remove the specific brain lesion will render him seizure free, with no need to take medications and able to lead a normal life.
Four thousand miles away in the UK, 2 MD PhDs, Sophie Adler and Konrad Wagstyl, have been working on a project over the past four years to develop an AI application to detect FCD. The project concluded last year (2022) with a paper published in Brain describing Multi-centre Epilepsy Lesion Detection (MELD), a robust and interpretable machine-learning algorithm for automated detection of FCD. They created their FCD-detecting algorithm with a cohort of 618 patients with FCD-related epilepsy from 22 epilepsy centers worldwide. Their neural network was trained and cross validated on 50% of the total cohort and tested on the remaining 50%, as well as at 2 independent test sites. MELD is able to provide personalized patient reports identifying the location of predicted lesions alongside their imaging features.
Sadly, the work sits in Brain magazine. Rather than leave it there, let’s consider how the Chang Method could translate this research from the bench at University College London to bedsides around the world.
The first step is to implement the UK researchers’ FCD detection application, MELD, as an edge cloud application. The second step it to deploy the application to the edge zones in all the institutions that participated in the original work. The figure below shows the 22 sites that participated. The MELD application could be used both for detection in real-time or accessing historical MRIs stored in local PACS applications.
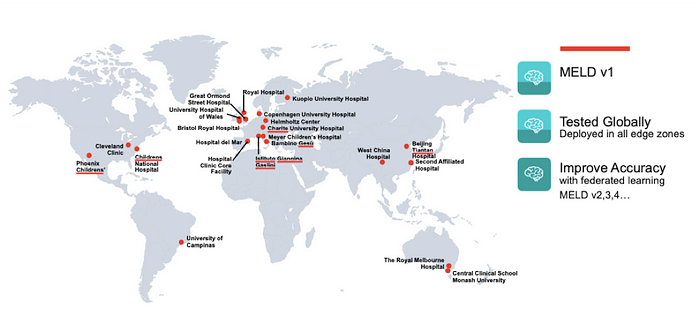
Finally, federated learning techniques are used to further improve accuracy based on a much larger data set, and of course, ultimately deployed to many more locations, all while preserving patient privacy.
The MELD FCD detection application is of course just one of 100s or 1000s of potential AI applications that can be deployed across this system to the benefit of all areas of pediatric medicine — from cardiology, radiology and endocrinology, to neurology, orthopedics, and neonatology. We have the technology to create real-time privacy preserving applications, which transform healthcare for all children, including those who are not socially or geographically lucky. Why wouldn’t we implement the Chang Method and move many more AI research projects from the bench to the bedside?
If you’d like to stay up to date on our progress to the moon, register for the newsletter at www.pediatricmoonshot.com, follow us on LinkedIn, subscribe to the Pediatric Moonshot podcast, listen to the Spotify playlist, and subscribe to the Pediatric Moonshot Youtube channel.
Many thanks for extensive editing by Laura Jana, Pediatrician, Social Entrepreneur & Connector of Dots; Leanne West, Chief Engineer of Pediatric Technology at Georgia Tech. Special thanks to Alberto Tozzi, Head of Predictive and Preventive Medicine Research Unit at Ospedale Pediatrico Bambino Gesù for the translation to Italian
